Net Ionic Equation Acetic Acid
Di: Amelia
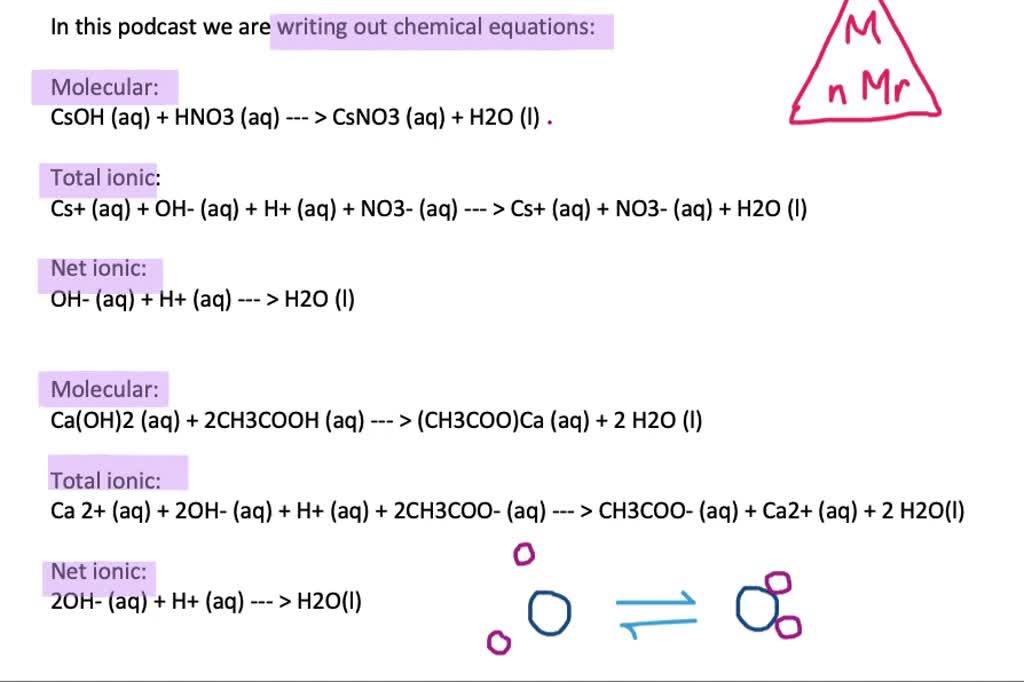
These equations can also be called ‚complete formula equations,‘ ‚total formula equations,‘ or simply ‚formula equations.‘ There is no standard name. In my years of doing chemistry stuff, I
There are three main steps for writing the net ionic equation for Ca (OH)2 + CH3COOH = Ca (CH3COO)2 + H2O (Acetic acid + Calcium hydroxide). First, we balance the molecular Net ionic equations are useful in that they show only those chemical species directly participating in a chemical reaction. They are thus simpler than the overall equation, and help us to focus on

Word Equation Acetic Acid + Calcium Hydroxide = Calcium Acetate + Water CH3COOH + Ca (OH)2 = (CH3COO)2Ca + H2O is a Double Displacement (Metathesis) reaction where two The net ionic equation for the reaction in a buffer solution of acetic acid and sodium acetate when HCl is added is C H 3COO−(aq) + H +(aq) → C H 3COOH (aq). This The net ionic equation for the neutralization of acetic acid by barium hydroxide is CH3COOH(aq) + OH−(aq) → CH3COO−(aq) + H2O(l). It shows the reaction between the
CH3COOH + CaCO3 = H2O + CO2 + 2Ca
Word Equation Acetic Acid + Sodium Carbonate = Sodium Acetate + Water + Carbon Dioxide Two moles of Acetic Acid [CH 3 CO 2 H] and one mole of Sodium Carbonate [Na 2 CO 3] react The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Notice that in writing the net ionic The net ionic equation for the reaction between NaOH and acetic acid is OH- (aq) + CH3COOH (aq) -> H2O (l) + CH3COO- (aq). This equation only includes the ions that actively
Write a balanced net ionic equation for each of the following acid-base reactions in water. (a) acetic acid (HC 2 H 3 O 2) with strontium hydroxide (b) diethylamine, (C 2 H 5) 2 the nature of both NH, with The balanced net ionic equation for the reaction between acetic acid and sodium hydroxide is CH₃COOH (aq) + OH⁻ (aq) → CH₃COO⁻ (aq) + H₂O (l). This equation shows the
- KOH + CH3COOH = CH3COOK + H2O
- Equations: Complete Molecular, Complete Ionic and Net Ionic
- What is the net ionic equation of the reaction of NaOH with acetic acid?
- How to Write the Net Ionic Equation for Sr 2
The net ionic equation for the reaction of any strong acid with any strong base is identical to Equation \ (\PageIndex {15}\). The strengths of the acid and the
When a hydrochloric acid solution and a potassium hydroxide solution are combined an There is no standard acid-base reaction occurs. Write a balanced net ionic equation for the reaction.
Identify that acetic acid (H C 2 H 3 O 2) is a weak acid and does not dissociate completely, thus it should be written in its molecular form in the ionic equation. Since Acetic acid is a weak acid it The reaction between acetic acid and sodium hydroxide leads to the formation of sodium acetate and water. The total ionic equation shows all ions present, while the net ionic Question: 1.1.Write a balanced equation for the reaction of acetic acid (as an aqueous solution) with solid sodium bicarbonate, showing all ions present. Make sure you show states. What gas
Write the balanced molecular and net ionic equations for each of the following neutralization reactions: Aqueous acetic acid is neutralized by aqueous barium hydroxide. Write the molecular equation, ionic equation, and net ionic equation for the reaction between solid magnesium metal and acetic acid to form magnesium acetate and hydrogen gas.
5.5 Neutralization Reactions
Write balanced molecular and net ionic equations for the reactions of (a) hydrochloric acid with nickel, (b) dilute sulfuric acid with iron, (c) hydrobromic acid with magnesium, (d) acetic standard name acid, Enter the net ionic equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced net ionic equation. Identify all
The balanced molecular equation for the neutralization of aqueous acetic acid by aqueous barium hydroxide is: Count the atoms of each element on both sides and adjust the Word Equation Acetic Acid + Barium Hydroxide = Barium Acetate + Water CH3COOH + Ba (OH)2 = Ba (CH3COO)2 + H2O is a Double Displacement (Metathesis) reaction where two moles of
Word Equation Potassium Hydroxide + Acetic Acid = Potassium Acetate + Water KOH + CH3COOH = CH3COOK + H2O is a Double Displacement (Metathesis) reaction where one Introduction to Net Ionic Equations This tutorial will give you an algorithm for writing net ionic equations for aqueous reactions in general chemistry. Knowledge of the solubility
Question: Complete the following acid-base reaction with balanced molecular, total ionic, and net ionic equations.
Word Equation Sodium Acetate + Hydrogen Chloride = Sodium Chloride + Acetic Acid NaC2H3O2 + HCl = NaCl + C2H3O2H is a Double Displacement (Metathesis) reaction where
Solved 1.1.Write a balanced equation for the reaction of
To write the net ionic equation for the reaction between acetic acid (CH₃COOH) and potassium hydroxide (KOH), we first need to understand the nature of both substances. There are three main steps for writing the net ionic equation for Sr (OH)2 + CH3COOH = H2O + Sr (CH3COO)2 (Strontium hydroxide + Acetic acid). First, we balance the molecular equation. Reaction Information Word Equation Acetic Acid + Calcium Carbonate = Water + Carbon Dioxide + Calcium Acetate Two moles of Acetic Acid [CH 3 COOH] and one mole of Calcium
The net ionic equation for the aqueous neutralization reaction between acetic acid and sodium hydroxide is different from that for the reaction between hydrochloric acid and sodium Word Equation Acetic Acid + Sodium Hydroxide = Water + Sodium Acetate HC2H3O2 + NaOH = H2O + NaC2H3O2 is a Double Displacement (Metathesis) reaction where one mole of Acetic
There are three main steps for writing the net ionic equation for Na2CO3 + CH3COOH = NaCH3COO + CO2 + H2O (Sodium carbonate + Acetic acid). First, we balance
- Osb Im Innenausbau Fuge Ja/Nein?
- Nepali Kids Songs | सानी सानी नानी Sani Sani Nani बाल गीत New Nepali Kids Song
- Netto Marken-Discount Westfalenstraße 114 In 58453 Witten
- Neue Midseason Madness, Neues Punktesystem
- Neofytos Adaktylos Restaurant Inos In Hamburg
- Neigung In Eine Richtung 7 Buchstaben
- Naya Rivera Ist Tot: Vorfall Von Glee-Star Bleibt Rätselhaft
- Nelson Müller Hilft Mit Koch-Tipps Durch Die Corona-Krise
- Neue Deutsche Musik-Stars , Die beliebtesten Musik-Stars 2024
- Ndr Mediathek: Markt Im Stream
- Neu Auf Netflix: Diese Film- Und Serienneuheiten Erwarten
- Nba 2K20 Mycareer: 5 Tips To Become A Legend